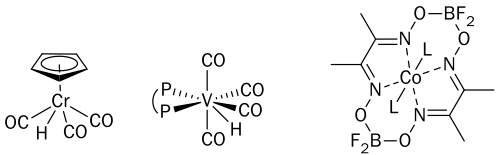
Tin hydrides (i.e., Bu3SnH) have traditionally been used as stoichiometric H• donors in radical reactions. The byproducts from such tin hydrides are, however, toxic and difficult to remove, precluding the use of these reactions on industrial scale. We have shown that transition-metal hydrides (e.g., 1, 2, and 3) can be used as replacements for tin hydrides, and some can be used catalytically under H2, resulting in radical reactions that are green and atom-economical (generating almost no waste).
Transition-metal hydrides with reduced M-H bond strengths have enhanced reactivity, and can generate radicals directly from olefins by H• transfer. By measuring the physical properties of such metal hydrides (e.g., the strength of their M-H bonds, the rates at which they transfer H• to typical substrates, and the mechanisms of their reaction with H2), we can predict which hydrides will be effective catalysts for radical reactions.