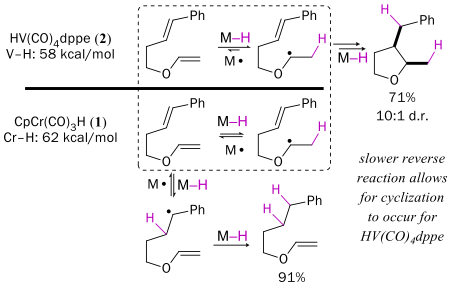
The Cr-H bond in CpCr(CO)3H is 62 kcal/mol, whereas the V-H bond in HV(CO)4dppe is only 56 kcal/mol. The difference in reactivity is large; for example, H• transfer to styrene is 10.6 x faster with vanadium. However, the V-H bond is so weak that V• does not reform the hydride by cleaving H2, so the vanadium system is not catalytic.
The M-H bond strength affects the outcome of radical cyclization reactions. For example, both CpCr(CO)3H and HV(CO)4dppe transfer H• to enol ethers, but the reverse reaction (transfer of H• back to the metal) is faster for Cr than for V. As a result alpha-alkoxy radicals generated from 2 cyclize, but those generated from 1 do not live long enough to do so.